Microbes ‘MacGyver’ membrane transport
A general concept in biology is that cells use two different systems to transport substances across their membrane: selective pores, which allow passive transport driven by a concentration gradient, and active transport complexes, which use energy to transport substances against a gradient. Now scientists from the University of Groningen, together with colleagues from Germany and Spain, have described a system that combines a pore and a transporter to import potassium ions into cells against an extreme concentration gradient. The results were published in Nature Communications on 26 November.
Cristina Paulino, head of the cryo-EM unit at the Department of Structural Biology at the University of Groningen, is excited: ‘This could very well become textbook stuff!’ The novel transport system combines two worlds that were seen as separate in many ways. Passive pores and active transporters use different mechanisms, have a different evolutionary history and are mostly studied by different groups of scientists, each with their own nomenclature and conferences. Two worlds that were believed to be mutually exclusive. ‘We have shown that it is not always so black and white.’

High affinity
Together with her colleague Inga Hänelt from Goethe University Frankfurt (Germany), Paulino has identified a chimera between a pore and a transporter which is present in the gut bacteria E. coli and a few related species. Like MacGyver, these bacteria have taken the best parts of these two transport systems and combined them to form something new. ‘It is really fascinating to discover that nature can do this’, says Paulino.
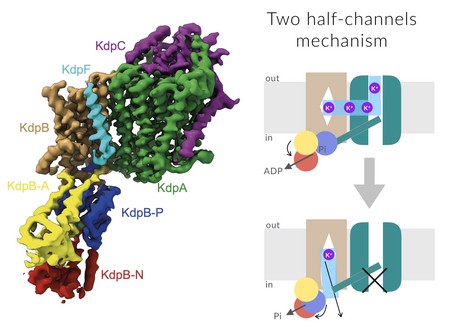
The transport system, named KdpFABC, is used to transport potassium ions into the cell. It is only activated in extreme cases, e.g. when the concentration inside the cells is are about 10,000 times higher than the concentration outside. Paulino: ‘In this situation, where the concentration outside is so very low, you need extremely high affinity to bind the potassium ions, and the system needs to overcome a very high energy barrier to import the potassium ions.’
Channel architecture
Pores usually have high selectivity and affinity, but only active transport can overcome the energy barrier. So how can KdpFABC do both? Scientists realized the answer would lie in the structure of this protein complex, which comprises four subunits. Last year, a structure was determined using X-ray diffraction. This confirmed that the complex contained one subunit with a channel architecture and another with an active transporter architecture. ‘However, we felt that the transport mechanism that was proposed based on this structure was not convincing. Something was missing.’

Paulino and her PhD student Lisa Hielkema, who is joint first author of the paper, therefore had another look at the structure of the protein complex. They used cryo-EM, a technique that uses a special electron microscope that can resolve details to a few angstrom. Thousands of proteins are imaged, and the images are combined. ‘We used inhibitors to make sure all the complexes were stabilized in the same state, as they change shape during the transport process.’ Despite the stabilizers, the results showed the complex in two different shapes. This was fortunate, however, because the two shapes showed two stages in the transport process.
Unique structure
The channel and the transporter subunit were clearly visible in Paulino and Hielkema’s analysis, but the structure of the channel subunit did not change, and it did not open to the inside. By contrast, Paulino and her colleagues could identify a set of tunnels that allow potassium ions to enter the channel from the outside, bridge to the transporter unit and from there, pass through another tunnel into the cell.
The transport mechanism that was derived from the structure is unique, Paulino explains. ‘The pore binds potassium ions on the outside with very high affinity. These potassium ions flow into the transport complex, which causes a change in conformation and is driven by the use of energy.’ The transport subunit uses ATP, the general energy carrier of the cells, to ‘fuel’ the conformational change, which opens up a tunnel to the interior of the membrane. The change in conformation also reduces the affinity for potassium ions, so the ions can be released inside the cell.
Dogma
‘The system uses a channel which evolved to have a very high selective affinity for potassium ions with a pump that evolved for transport against a very high energy barrier’, says Paulino. Thus, the system blurs the boundaries between pores and active transporters. ‘Our finding goes against the dogma that these two systems are mutually exclusive. We describe a totally new mechanism that uses old concepts in a unique combination.’
Reference: C. Stock, L. Hielkema, I. Tascón, D. Wunnicke, G. T. Oostergetel, M. Azkargorta, C. Paulino & I. Hänelt: Cryo-EM structures of KdpFABC suggest a K+ transport mechanism via two inter-subunit half-channels. Nature Communications, 26 November 2018
| Last modified: | 28 November 2018 5.00 p.m. |
More news
-
10 June 2024
Swarming around a skyscraper
Every two weeks, UG Makers puts the spotlight on a researcher who has created something tangible, ranging from homemade measuring equipment for academic research to small or larger products that can change our daily lives. That is how UG...
-
21 May 2024
Results of 2024 University elections
The votes have been counted and the results of the University elections are in!

