Green chemistry now even greener
Three years ago, University of Groningen chemist Ben Feringa presented a cleaner method to make compounds that are important for pharmaceuticals and more. He has now come up with a follow-up that is cleaner still. Nature Communications published the new technique on 2 June.
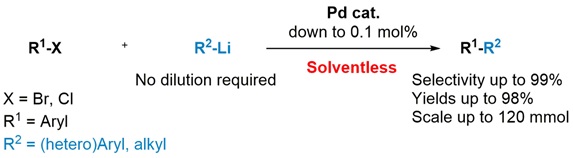
Organic chemistry tends not to be particularly clean. Reactions often take place in organic solvents such as hydrocarbons or toluene, and high temperatures or toxic metals may be required too. The main challenge is forming carbon-carbon bonds. In 2013, Ben Feringa and his colleagues described a greener method to form these bonds, using what are known as organolithium reagents in combination with a palladium catalyst.
‘Organolithium fell into obscurity 30 years ago. They thought that it was too reactive to be of use,’ says Feringa. But it proved to work well in combination with a palladium catalyst. Reactions that took 48 hours at 90 degrees Celsius using standard industrial methods now took one hour at 20 degrees. Organic solvents were still needed, however.
But that was 2013, and Feringa has made significant progress since then. ‘We have adjusted the catalyst somewhat, and have now carried out the reactions without extra solvents.’ In Nature Communications, the Groningen researchers explain that the carbon-carbon bonds still form and, what is more, the reaction now occurs in 10 minutes. ‘At first we added all the reagents slowly, as is customary, but that proved not to be necessary.’
The result is that the quantity of pollutant (organic solvents and metals in particular) used is five to ten times lower than with standard techniques. ‘Furthermore, we have shown that this reaction can be scaled up to larger quantities.’ The carbon-carbon bonds are particularly important for producing compounds that are used by the pharmaceutical industry or for LEDs, for instance.
Reference: Erik B. Pinxterhuis, Massimo Giannerini, Valentín Hornillos & Ben L. Feringa: Fast, Greener and Scalable Direct Coupling of Organolithium Compounds With No Additional Solvents. Nature Communications 2 June 2016. DOI: 10.1038/ncomms11698
| Last modified: | 28 October 2016 11.46 p.m. |
More news
-
10 June 2024
Swarming around a skyscraper
Every two weeks, UG Makers puts the spotlight on a researcher who has created something tangible, ranging from homemade measuring equipment for academic research to small or larger products that can change our daily lives. That is how UG...
-
21 May 2024
Results of 2024 University elections
The votes have been counted and the results of the University elections are in!

