Nuclear fission (natural reactor)
History
In 1972, routine measurements on uranium mined in Oklo (Gabon, Africa) showed a 235U concentration lower than the expected. Further investigations discovered uranium ore with a 235U concentration as low as 0.44%, which is almost 40% below the normal abundance of 0.72%.
As it turned out, it were the remnants of an extinct nuclear fission reactor, where self-sustaining nuclear reactions have occurred about 2 billion years ago. At that time, the fissile isotope 235U made up about 3.1% of the natural uranium, which is comparable to the amount used in some of today's reactors.
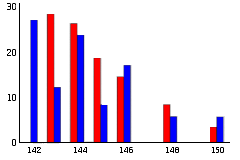
Neodymium and other elements were found with isotopic compositions different from what is usually found on earth. For example, Oklo contained less than 6% of 142Nd while natural neodymium contains 27%. However, Oklo contained more of 143Nd. The isotopic composition matched that produced by the fission of 235U (see Figure).
Related concepts
| Last modified: | 10 April 2024 11.00 a.m. |
