
PI: Frank A. Kruyt, PhD

Position: Professor
Contact: email
Phone number: +31 (0)50 361 5531
Publications: Pubmed
Biography
Frank A.E. Kruyt obtained his PhD in the field of developmental biology at the Hubrecht Laboratory in Utrecht, the Netherlands (Prof. dr. S.W. de Laat/dr. P.T. van der Saag). His postdoctoral research was dedicated to the study of bone marrow failure and cancer prone disorder Fanconi Anemia (FA), cloning new FA genes and elucidating their function, at the VU University in Amsterdam, the Netherlands(with Prof. dr. H. Joenje) and Baylor College of Medicine, Houston, Tx, USA (with Prof. dr. H. Youssoufian). Intrigued by cancer biology and therapy he entered the field of oncology, exploring the possibility of using the FA pathway and apoptosis as targets for cancer therapy at the department of Medical Oncology at the VUMC, Amsterdam, the Netherlands. Currently, as professor in the field of Experimental Oncology at the department of Medical Oncology, UMCG, Groningen, his group is continuing work on targeting cell death regulatory mechanisms and stress responses in cancer. Importantly, recognizing tumor cell heterogeneity, the role and mechanisms of tumor cell differentiation (CSC and EMT) in tumor aggressiveness and targeted therapy are being studied with a focus on aggressive brain tumors (glioblastoma) and lung cancer. He has obtained funding amongst others from the Netherlands Organization for Scientific Research (MWO), Dutch Cancer Society (KWF) and Top Institute Pharma (TIPharma). He is editorial board member of Biochemical Pharmacology and Apoptosis and review editor of Frontiers in Molecular and Cellular Oncology.
Research focus
1. Targeted therapy: Cell death and ER stress
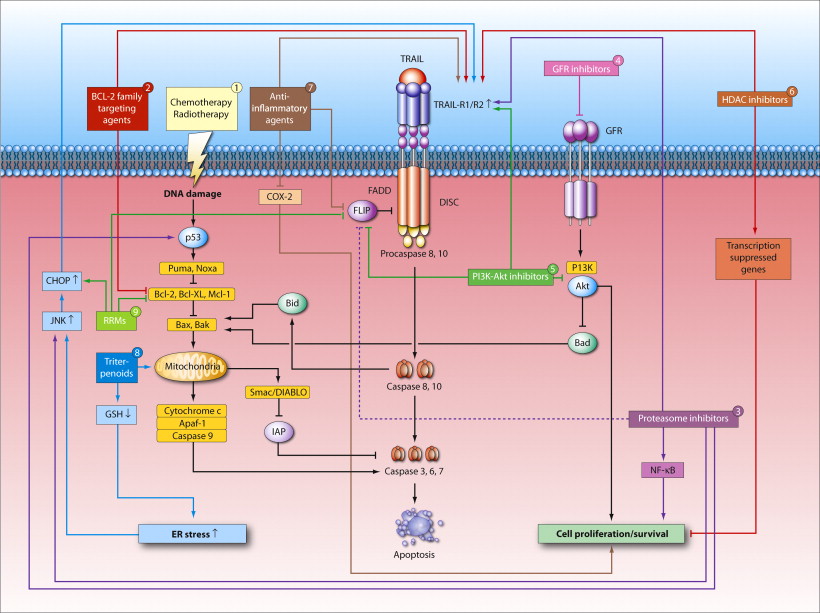
The selective activation of programmed cell death or apoptosis in cancer cells is one of the main objectives in this research line. Particularly, mechanisms of TRAIL receptor signaling are examined in order to optimize clinical benefit of TRAIL receptor agonists in lung cancer. ER stress/the unfolded protein response (UPR) and autophagy are also important for maintaining cellular homeostasis and survival. Underlying mechanisms and therapeutic value are examined in glioblastoma (stem cell) models.
2. Cancer stem cells and Epithelial-Mesenchymal Transition (EMT): novel therapeutic opportunities
The tumor microenvironment (TME) plays a major role in the development and progression of cancer. This not only includes cancer prone conditions such as shortage in nutrients and oxygen, but also the active secretion of pro-tumorigenic growth factors/cytokines from normal cells like fibroblasts, immune cells and endothelial cells within or surrounding the tumor. In my group, we study the effect of the TME on cancer stem cells (CSC) and differentiation status of tumor cells, including epithelial-to-mesenchymal transition (EMT), known to determine tumor growth and invasive/metastatic activity as well as resistance to therapy. Identified mechanisms are explored for use as new targets for therapy.
For further information regarding these research projects, please contact Frank Kruyt
Group Members
- Margot de Looff - PhD student
- Natalia Peñaranda Fajardo - PhD student
- Siobhan Conroy - PhD student
- Win Sen Heng - PhD student
- Yuanke Liang - MD/PhD student
Selected publications
- Kahlert UD, Joseph JV, Kruyt FA (2017). EMT- and MET-related processes in nonepithelial tumors: importance for disease progression, prognosis, and therapeutic opportunities. Mol Oncol. 11:860-77.
- Peñaranda Fajardo NM, Meijer C, Kruyt FA (2016). The endoplasmic reticulum stress/unfolded protein response in gliomagenesis, tumor progression and as a therapeutic target in glioblastoma. Biochem Pharmacol. 118:1-8.
- Joseph JV, Conroy S, Pavlov K, Sontakke P, Tomar T, Eggens-Meijer E, Balasubramaniyan V, Wagemakers M, den Dunnen WF, Kruyt FA (2015). Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1α-ZEB1 axis. Cancer Lett. 359:107-16.
- Joseph JV, Conroy S, Tomar T, Eggens-Meijer E, Bhat K, Copray S, Walenkamp AM, Boddeke E, Balasubramanyian V, Wagemakers M, den Dunnen WF, Kruyt FA (2014). TGF-β is an inducer of ZEB1-dependent mesenchymal transdifferentiation in glioblastoma that is associated with tumor invasion. Cell Death Dis. 5:e1443.
- Azijli K, Yuvaraj S, Peppelenbosch MP, Würdinger T, Dekker H, Joore J, van Dijk E, Quax WJ, Peters GJ, de Jong S, Kruyt FA (2012). Kinome profiling of non-canonical TRAIL signaling reveals RIP1-Src-STAT3-dependent invasion in resistant non-small cell lung cancer cells. J Cell Sci. 125:4651-61.
- Kruyt FA (2008). TRAIL and cancer therapy. Cancer Lett. 263:14-25.
- Bröker LE, Kruyt FA, Giaccone G (2005). Cell death independent of caspases: a review. Clin Cancer Res . 11:3155-62.
- Bröker LE, Huisman C, Span SW, Rodriguez JA, Kruyt FA*, Giaccone G*(2004). Cathepsin B mediates caspase-independent cell death induced by microtubule stabilizing agents in non-small cell lung cancer cells. Cancer Res. 64:27-30.
- Waisfisz Q, de Winter J, Kruyt FA, Dijkmans L, Schepers R, Arwert F, Youssoufian H, Hoatlin M, and Joenje H (1999). A physical complex of the Fanconi anemia proteins FANCG/XRCC9 and FANCA. Proc Natl Acad Sci U S A. 96:10320-5.
- Kruyt FA and Youssoufian H (1998). The Fanconi anemia proteins FAA and FAC function in different cellular compartments to protect against cross-linking agent cytotoxicity. Blood. 92:2229-37.
- Lo Ten Foe JR, Rooimans MA, Bosnoyan-Collins L, Alon N, Wijker M, Parker L, Lightfoot J, Carreau M, Callen DF, Savoia A, Cheng NC, van Berkel CGM, Strunk HP, Gille JJP, Pals G, Kruyt FA, Pronk JC, Arwert F, Buchwald M, Joenje H (1996). Expression cloning of a cDNA for the major Fanconi anemia gene, FAA. Nature Genet. 14:320-3.
- Kruyt FA , Dijkmans LM, van den Berg, Joenje H (1996). Fanconi anemia genes act to suppress a cross-linker-inducible p53-independent apoptosis pathway. Blood. 87:938-48.
| Last modified: | 10 October 2017 10.39 a.m. |
