Light-controlled drug carrier brings precision therapy closer
If you want to treat a tumour, the chemotherapeutic drug has to travel through the patient’s entire body, potentially causing many side effects in healthy tissues. Scientists at the University of Groningen and the University Medical Center Groningen have previously explored ways to activate drugs at the site where they are needed using light-controlled switches. They have now produced a light-controlled ‘cage’ that ‘opens’ to deliver the drug to where it is needed. The cage was designed using a computer and subsequently created using synthetic chemistry. A paper on this designer cage was published in Angewandte Chemie on 19 February 2022 as a ‘Very Important Paper’.
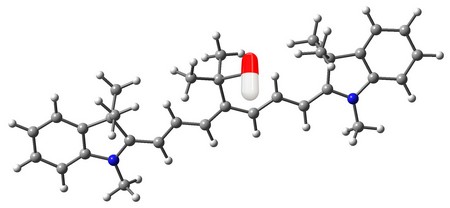
Photo cages are molecular tools that are used to deliver drugs. They are not really cages or boxes but large molecules that are attached to a drug. While bound to a cage, the drug is inactive. When a cage is irradiated with light of the correct wavelength (colour), the drug is released and becomes active. ‘The challenge until now has been to produce cages that respond to near-infrared light, since this can penetrate the body much better than UV or visible light,’ explains George Alachouzos. Traditionally, such new cages are designed by changing the wavelength at which existing cages open to the near-infrared.

Electron density
While working on his PhD thesis in organic chemical synthesis in the US, Alachouzos became interested in photo switches and cages. ‘I started to study papers from the laboratories of Ben Feringa and Wiktor Szymanski on drugs that could be switched on,’ he explains. Feringa, Professor of Organic Chemistry at the University of Groningen, was one of the recipients of the Nobel Prize for Chemistry in 2016 for his work on a different class of light-activated molecular tools: light-driven motors. Szymanski is a long-time collaborator of Feringa and associate professor at the Medical Imaging Center, University Medical Center Groningen.
While studying the literature on photocages, Alachouzos noticed something: the point where the drug was released always seemed to show a particular electron behaviour. In all these cages from the literature, electrons are shared between the different atoms in the cage molecules. Alachouzos: ‘This means that the distribution, and therefore the electron density, is sometimes unevenly spread across the molecule. The energy from the light that was used to unlock these literature cages appeared to increase the electron density at the point where they were connected to the drug.’ An electronic orbital lobe would show up in the electron density where there wasn’t one before. ‘The observation was that this “node-to-lobe” change drives the opening of these cages. My hypothesis was that any dye that shows this change in electron configuration upon irradiation could be made into a photocage.’
Grant
He contacted Feringa and Szymanski to discuss his idea. Together, they wrote a successful grant application for the European Molecular Biology Organization, enabling Alachouzos to come to Groningen and test his hypothesis. ‘First, I made a catalogue of dyes that absorb near-infrared light. Then, I used the Peregrine supercomputer at the Center for Information Technology to see which of these dyes could be irradiated with near-infrared light to cause the node-to-lobe change.’
The procedure yielded a number of candidates. One of them was from a well-known dye family that is used in fluorescence experiments in animals and humans and even in the clinic: Cy7. ‘It responds to light of 736 nanometres, which can penetrate about three centimetres into the body, and appeared ideal for our purpose, especially since it was already clinically applied.’ The next step was to adapt the dye to turn it into a photocage. This required adding a linker that could attach to a drug near the point where the node-to-lobe change occurs. ‘It took around five months to do this. There was no way to simply add this bit to Cy7; we had to build the entire molecule from scratch.’
Dutch delicacy
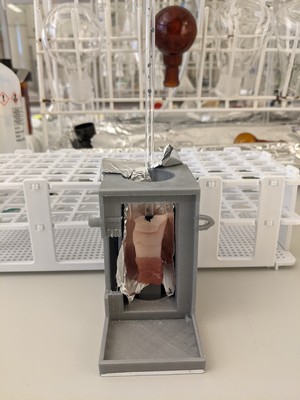
The modified photocage Cy7-PPG was bound to acetic acid, as a placeholder for future drugs. Then it was time to test the cage. It worked fine in the laboratory, thus proving Alachouzos’ hypothesis. The next step was to test it under more ‘realistic’ conditions. ‘Doing this in humans or even animals would require lots of safety studies because the Cy7 had been modified. But walking across the Groningen food market, I got another idea.’
He used a Dutch delicacy, Hollandse Nieuwe (salted raw herring), as his experimental model. He inserted a small tube into the centre of a 1 cm thick cut of herring meat and irradiated this with near-infrared light through 0.5 cm of the fish meat. ‘We found a very high release rate of the acetic acid, 86 percent versus if we had irradiated the sample directly, rather than through the herring meat. This signifies that near-infrared light passes almost completely unhindered through the fish!’
His co-supervisor Wiktor Szymanski then suggested using a second model that would resemble human skin, muscle, and fat tissue more closely. The inspiration for this was also taken from Dutch cuisine: an uncooked speklapje (slice of pork belly). Again, the light penetrated the meat and released the acetic acid from the cage with a 78 percent efficiency.

Specific-purpose cages
‘We now have a proof of principle that it is possible to design a photocage in silico,’ says Alachouzos. ‘This will make it much faster to create new, specific-purpose cages.’ He is now working with several Master’s and PhD students towards attaching relevant drugs to the next generation of Cy7 cages. ‘We aim to use drugs that must act locally, such as antibiotics, anti-inflammatory drugs, or chemotherapeutic drugs, and the results so far are very promising.’
Alachouzos is one year into his two-year postdoctoral researcher post and hopes to obtain more results. ‘Working at the interface of Ben Feringa’s synthetic organic chemistry group and Wiktor Szymanski’s more clinically oriented group and with our UMCG partners has enabled the publication of this work – it wouldn’t be possible at most other top institutes and without this excellent team of co-authors! This really is the best environment for scientific research that spans from the extremely fundamental to the extremely applied.’
Reference: Georgios Alachouzos, Albert M. Schulte, Anirban Mondal, Wiktor Szymanski and Ben L Feringa: Computational Design, Synthesis, and Photochemistry of Cy7PPG, an Efficient NIR-Activated Photolabile Protecting Group for Therapeutic Applications. Angewandte Chemie, 19 February 2022
| Last modified: | 28 November 2024 3.33 p.m. |
More news
-
20 December 2024
Three FSE researchers receive NWO M1 grant
Dr. Antonija Grubišić-Čabo, Dr. Robbert Havekes and Prof. Jan Komdeur receive an NWO M1 grant
-
19 December 2024
NWO ENW-XL million grants for RUG research projects
Four researchers from the Faculty of Science and Engineering (UG) receive NWO grants of 3 million euros for their research projects.
-
19 December 2024
Jacquelien Scherpen honoured with Hendrik W. Bode Lecture Prize 2025
For her achievements in the scientific development of control systems and engineering, Rector Jacquelien Scherpen has received the 2025 Hendrik W. Bode Lecture award from the IEEE Control Systems Society (CSS).

