Largest ever genetic study of malaria mosquito
The largest ever genetic study of mosquitoes reveals the movement of insecticide resistance between different regions of Africa and finds several rapidly evolving insecticide resistance genes. Reported in Nature on 29 November, this genetic resource will be used to develop new tools for monitoring resistance and managing insecticide use, and for designing novel control methods.

Malaria is transmitted by mosquitoes and rising resistance to insecticides is hampering efforts to control the disease. The study led by researchers from the Wellcome Trust Sanger Institute and their collaborators including Michael C. Fontaine from the Groningen Institute for Evolutionary Life Sciences (GELIFES) of the University of Groningen also discovered that wild mosquitoes collected in Africa were genetically far more diverse than had been thought. This helps to explain how mosquitoes evolve insecticide resistance so quickly.
Diverse
To understand how mosquitoes are evolving, researchers working with the Anopheles gambiae 1000 genomes project sequenced the DNA of 765 wild Anopheles mosquitoes. These were taken from 15 locations across eight African countries , creating the largest data resource on natural genetic variation for any species of insect. They then examined each of the mosquito genomes.
The researchers revealed that the Anopheles gambie mosquitoes are extremely genetically diverse compared with most other animal species. High genetic diversity enables rapid evolution and the study found 52 million small differences amongst the mosquito genomes.
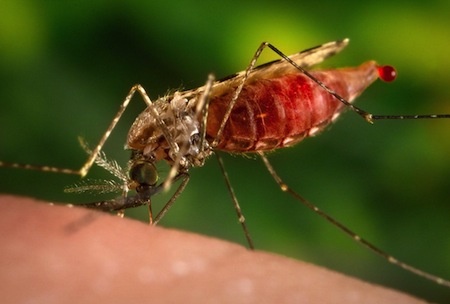
Michael Fontaine, contributing authors from the University of Groningen, said: ‘Such extraordinary level of genetic diversity observed in these mosquitoes certainly explains their abilities to adapt to rapidly changing environments and to the vector control strategies. From a more fundamental perspective, the resources produced in this project and those coming in a near future will be invaluable to study how Anopheles gambiae has recently diverged from its closest relative Anopheles coluzzii, revealing key processes about how species can diverge despite high level of genetic exchange between them.’
Gene drive
New strategies to control mosquitoes are being developed that use ‘gene drive’– using the latest Crispr/Cas 9 genetic tools to make mosquitoes infertile or unable to carry the malaria parasite. However, this technology requires an exact match with any targeted gene. The researchers found that gene drive is unlikely to work for most mosquito genes because they are too variable in nature, however they also used the data to highlight less variable targets that are potentially more suitable for gene drive based methods to control mosquitoes.

The mosquito genomes also revealed rapid evolution of several genes that had previously been implicated in insecticide resistance. Unexpectedly, the researchers discovered many previously-unknown genetic variants within those genes that could be causing insecticide resistance. Worryingly, they showed that these genetic variants for insecticide resistance were not only emerging independently in different parts of Africa, but were also being spread across the continent by mosquito migration.
Malaria
More than 200 million people are infected with the malaria parasite worldwide each year, which is transmitted by blood-sucking Anopheles mosquitoes. Malaria caused the deaths of around 429,000 people in 2015 with the majority of cases in sub-Saharan Africa.
Public health measures in Africa such as insecticide-treated bed nets and insecticide-spraying have helped reduce the numbers of malaria cases since 2000, but many mosquitoes have evolved resistance to insecticides. This is now threatening to derail malaria control in Africa.
Reference: The Anopheles gambiae 1000 Genomes Consortiu: mGenetic diversity of the African malaria vector Anopheles gambiae. Nature, 30 November 2017, DOI:10.1038/nature24995
Report: Wellcome Trust Sanger Institute.
| Last modified: | 19 December 2017 11.10 a.m. |
More news
-
30 January 2025
Highlighted papers December 2024 - January 2025
Read our highlighted papers from December/January: new insights into electronics from 2D materials, and into the protein clumps that cause Huntington's disease.
-
28 January 2025
Studying the universe to understand the world
By understanding the cosmos, we can better fathom the fundamentals of our world. That is the idea behind the research theme Fundamentals of the Universe, in which three institutes of the University of Groningen form a unique collaboration.
-
27 January 2025
Working on better AI (with a smaller budget than the US)
The US is going to invest a staggering amount of $500 billion in AI. At the University of Groningen, researchers are working on future-proof computing: more energy-efficient hardware and responsible AI that can collaborate with humans.

