Vitamin B3 gives Dirk Slotboom fifth Nature paper
By figuring out how bacteria manage to get their dose of vitamin B3 into the cell, Dirk Slotboom stumbled upon the competitive field of sugar transport. He also had a paper published in Nature Structural and Molecular Biology this week, his fifth in a Nature journal in under two years.

‘I’m interested in finding out how cells can take up small molecules from their surroundings’, explains Slotboom in his office in the Physics & Chemistry building. He also prefers to investigate new systems, which is why, just over a year ago, he set his sights on the PnuC transporter, which conveys vitamin B3 into cells. ‘We knew from research from years ago that this was a vitamin-B transporter. This led us to search genomic databases for related bacterial genes that might code for such a transporter.’
One of the specialties of Slotboom’s research group is elucidating the molecular structure of transport systems with x-ray crystallography . To do this you need crystals of the molecule you want to study. ‘But you never know whether a molecule will yield good crystals. So our approach is to find as many similar proteins as possible and see if one of them will crystallize.’
The gene search yielded a whole family of PnuC-like proteins, one of which, from the bacterium Neisseria mucosa, produced good crystals. ‘Once you have your crystals and the results of the x-ray diffraction measurement, you can immediately see the structure of the protein. That is one of the best moments you can have as a scientist!’
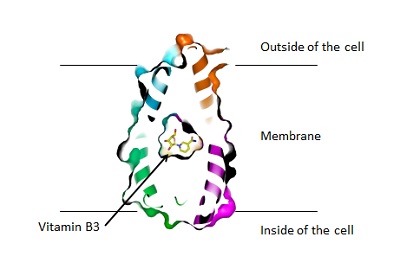
What Slotboom saw was a protein that wound itself around the cell membrane seven times. There was a striking symmetry to it with the first three segments looking very much like the last three. ‘In structure, that is: the amino acids that make up these segments were very dissimilar.’
The symmetrical structure reminded him of something he had seen before: a sugar transporter called SWEET. ‘That is actually an acronym for Sugar Will Eventually Efflux Transporter, as this protein can transport sugar both ways: into or out of the cell.’ The vitamin transporter has the same capabilities. ‘Although in nature, the vitamin is modified once it is inside the cell and does not come out again.’
The sugar transporter is present in cells lining the gut, which carry sugar from food into the bloodstream. It is also present in plant cells involved in the excretion of nectar. ‘That is a hot topic for plant breeders, so a lot is already known about the SWEET transporters.’
Just before Slotboom published the structure of his transporter, another group released the structure of a SWEET transporter. ‘But that was half the size, with just three membrane-spanning segments.’ In case you were wondering, it is indeed called ‘semi-SWEET’.

The vitamin-B3 transporter Slotboom described may have some useful applications. ‘The bacterium Haemophilus influenzae, which can cause meningitis, is totally dependent on this transporter for vitamin B3. So if we could block this transporter it would kill it. Now that we know the structure of the transporter, it does seem feasible.’ Another approach would be to design a toxic molecule that would be transported into the cell.
However, Slotboom and his team will probably focus on more fundamental matters. ‘We are discussing the practical applications with colleagues and companies, but what we want to do most of all is study the dynamics of this vitamin transport. Now that we have a snapshot of the structure, we want to see how it really works.’
The current paper is the fifth in a series that Slotboom has had published in the Nature journals in under two years. ‘There is no real secret to our success. We use a range of techniques to study new transport systems. The editors seem to like it’, says Slotboom.
The full list of Nature papers in which Slotboom was corresponding author.
1: Jaehme M, Guskov A, Slotboom DJ. Crystal structure of the Vitamin B3 transporter PnuC, a full-length SWEET homolog. Nature Struct Mol Biol . 2014 Epub 2014 Oct 8.
2: Slotboom DJ. Structural and mechanistic insights into prokaryotic energy-coupling factor transporters. Nature Rev Microbiol . 2014 Feb;12(2):79-87.doi: 10.1038/nrmicro3175. Epub 2013 Dec 23. PubMed PMID: 24362466.
3: Erkens GB, Hänelt I, Goudsmits JM, Slotboom DJ, van Oijen AM. Unsynchronised subunit motion in single trimeric sodium-coupled aspartate transporters. Nature . 2013 Oct 3;502(7469):119-23. doi: 10.1038/nature12538. PubMed PMID: 24091978.
4: Jensen S, Guskov A, Rempel S, Hänelt I, Slotboom DJ. Crystal structure of a substrate-free aspartate transporter. Nature Struct Mol Biol . 2013 Oct;20(10):1224-6. doi: 10.1038/nsmb.2663. Epub 2013 Sep 8. PubMed PMID: 24013209.
5: Hänelt I, Wunnicke D, Bordignon E, Steinhoff HJ, Slotboom DJ. Conformational heterogeneity of the aspartate transporter Glt(Ph). Nature Struct Mol Biol . 2013 Feb;20(2):210-4. doi: 10.1038/nsmb.2471. Epub 2013 Jan 20. PubMed PMID: 23334291
| Last modified: | 10 June 2015 2.56 p.m. |
More news
-
10 June 2024
Swarming around a skyscraper
Every two weeks, UG Makers puts the spotlight on a researcher who has created something tangible, ranging from homemade measuring equipment for academic research to small or larger products that can change our daily lives. That is how UG...
-
21 May 2024
Results of 2024 University elections
The votes have been counted and the results of the University elections are in!

