From sugar to coke bottle
We all know that a regular coke contains a lot of sugar. What we might not know is that it’s possible to make coke bottles from sugar-based polymers. Glucose can be turned into PEF, a green alternative to PET, which can be used to make fizzy-drink bottles. Biochemists from the University of Groningen have described a single enzyme that can mediate no fewer than three steps in the chemical process from glucose to PEF.

It’s a special enzyme, says Professor of Biochemistry Marco Fraaije. ‘We used to think that enzymes were very specific, mediating just one type of reaction and one substrate. That view has changed, but to find an enzyme that performs three reactions in a row on one substrate is fairly unique.’
Fraaije’s PhD student Willem Dijkman worked on the enzyme, and his group described it in a paper published online earlier this month by the journal Angewandte Chemie. ‘It’s an oxidizing enzyme, isolated from a soil bacterium’, Fraaije explains. His group was interested in the enzyme because it could potentially oxidize a compound called HMF ( Hydroxymethylfurfural ), which explains why the enzyme is called HMFO (HMF Oxide).
‘HMF is derived from sugars like glucose or fructose. Glucose is partially converted into HMF when it’s heated.’ It’s therefore present in most baked products. The interesting thing about HMF is that it can be oxidized into FDCA, a compound that forms the building blocks of the polymer PEF, a bioplastic. PEF has similar properties to PET, the stuff that plastic bottles and fleeces are made of.
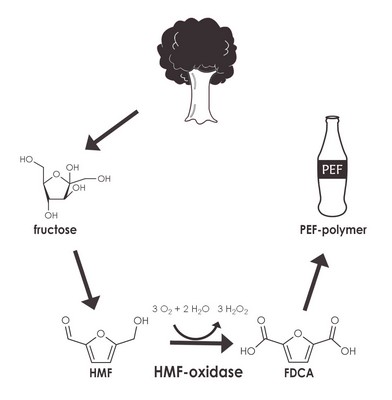
This is why the Dutch biotech company Corbion is interested in the biochemical process to make PEF. ‘We’ve formed a consortium with Corbion and other partners to work on the process’, says Fraaije. His group specifically looked at an HMF-oxidizing enzyme.
‘There aren’t many enzymes that can do this’, he explains. A small biotech firm that was recently taken over by Corbion had found a bacterium which could grow on HMF. ‘But the genes for the enzymes from this microorganism couldn’t be expressed in fast-growing lab strains like E. coli, which are needed to produce large quantities.’ So Fraaije looked for similar genes in large computerized DNA banks.
‘We found just ten or so, one of which turned out to be a winner.’ HMF-oxidizing enzymes are rare, probably because HMF is not found in nature, but the enzyme the Fraaije group worked on turned out to be extremely versatile. ‘We initially looked for the first step in the chain from HMF to FDCA. Then, to our surprise, we noticed that this enzyme was capable of catalyzing all three steps.’
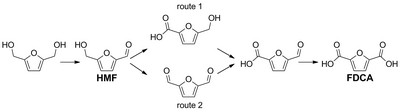
The first step involves oxidizing an alcohol group on the molecule, but the second one involves a different group, an aldehyde . ‘We expected our enzyme to be an alcohol oxidizer, so it was a bit of a surprise to see it also mediated the aldehyde oxidation steps.’
A closer look at the process provided the explanation. ‘It turns out that the aldehyde group can be hydrated to form a gem-diol , which is a special class of alcohol.’ It’s still impressive that the enzyme can handle three different substrates. In fact, the group was able to show that the enzyme needs only one compound to catalyze four reactions in a row, which is a new record for enzymes.

The purpose of the research programme was to gain more insight into the biochemical process in which sugars can be turned into FDCA by ‘natural’ means. ‘The entire process is also possible using standard chemical techniques, but these often require high pressure and temperatures, which takes a lot of energy.’ By contrast, the enzymatic process takes place at normal pressure and temperatures.
So will the enzyme described by Fraaije be used to make sugar-based coke bottles? ‘Probably not’, says Fraaije. ‘The process as we described it is too slow.’ However, Fraaije and his colleagues were not just in it for the bottles.
‘It’s given us new insight into the versatility of enzymes and the biochemical pathway from sugar to FDCA. We’re now working on the elucidation of the 3D structure of the enzyme, which will enable us to redesign it to increase its efficiency.’ And who knows what will come of that!
Enzyme-Catalyzed Oxidation of 5-Hydroxymethylfurfural to Furan-2,5-dicarboxylic Acid. Willem P. Dijkman, Daphne E. Groothuis and Prof. Dr. Marco W. Fraaije
Angewandte Chemie Article first published online: 6 May 2014, DOI: 10.1002/anie.201402904
| Last modified: | 14 December 2020 2.42 p.m. |
More news
-
20 December 2024
Three FSE researchers receive NWO M1 grant
Dr. Antonija Grubišić-Čabo, Dr. Robbert Havekes and Prof. Jan Komdeur receive an NWO M1 grant
-
19 December 2024
NWO ENW-XL million grants for RUG research projects
Four researchers from the Faculty of Science and Engineering (UG) receive NWO grants of 3 million euros for their research projects.
-
19 December 2024
Jacquelien Scherpen honoured with Hendrik W. Bode Lecture Prize 2025
For her achievements in the scientific development of control systems and engineering, Rector Jacquelien Scherpen has received the 2025 Hendrik W. Bode Lecture award from the IEEE Control Systems Society (CSS).

