
Quality
To ensure the best care of your samples, and the highest quality for your data, we only use validated standard operating procedures (SOPs). Special attention is given to measuring the DNA- or RNA quality before starting an experiment, and the performance of all laboratory steps are verified with in-process control experiments.
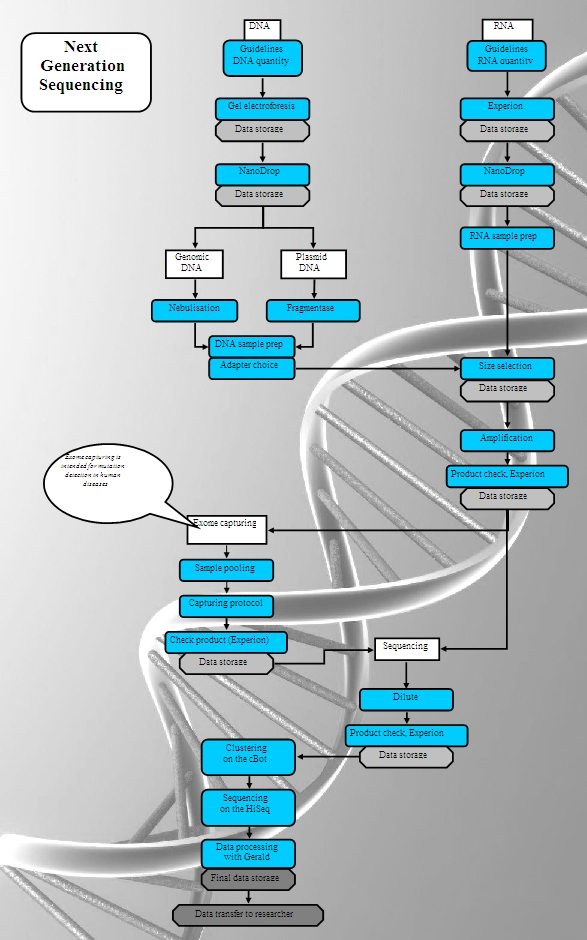
The Genome Analysis Facility works with a quality management system, which is integrated with SOPs and data storage features. The system is flexible and easy to adapt to new protocols and methods. This guarantees a constant high level of quality in a rapid developing field. Guidelines on the analysis of your starting material for genomic studies are given below.
Contact If you would like to discuss your project or ideas, or if you need further information about our facilities, please contact us, email
Guidelines
A good quality project for expression, genotyping or sequencing starts with good quality DNA or RNA. To serve researchers, the facility has prepared guidelines for the analysis of your starting material for genomic studies.
- Guidelines for analysis of RNA quantity and quality for array-based Illumina Expression studies
- Guidelines for analysis of DNA Quantity and Quality for Next Generation Sequencing Projects
- Guidelines for analysis of RNA Quantity and Quality for Next Generation Sequencing Projects
- Guidelines for analysis of DNA quantity and quality for Infinium and GoldenGate Projects
Complaints
To ensure the best care of your samples, and the highest quality for your data, we only use validated SOPs. Special attention is given to measuring the DNA or RNA quality before starting an experiment, and the performance of all laboratory steps are verified with in-process control experiments. The researcher is always told of unexpected results or delays.
Despite our best efforts, issues may arise regarding the quality of the final product. You should first try to resolve these in discussion with Mathieu Platteel, email, the contact person for our services. If you should require further assistance, please contact the Department of Genetic’s Quality Manager, Ms S. (Fia) te Velde, email.
| Laatst gewijzigd: | 12 augustus 2019 10:43 |
