Energy Conversion
Energy Conversion Lab focuses on the development of highly efficient, clean and flexible energy systems for a variety of applications including stationary power and heat production, fuel production and land, air and water transport.
System thermodynamics and fuel oxidation studies (combustion, electrochemical fuel oxidation) are at the core. Experimental and theoretical studies are carried out.
Combustion and electrochemical fuel conversion research is focused on the conversion of chemical fuels. Oxidation of new fuels, both sustainable (hydrogen, ammonia, biogas, biosyngas) and ‘new fossil’ (such as liquefied natural gas, LNG, as a transportation fuel) to assess their suitability for end-use equipment: efficiency, fitness-for purpose and environmental impact, i.e., to determine “the right fuel for the right job”.
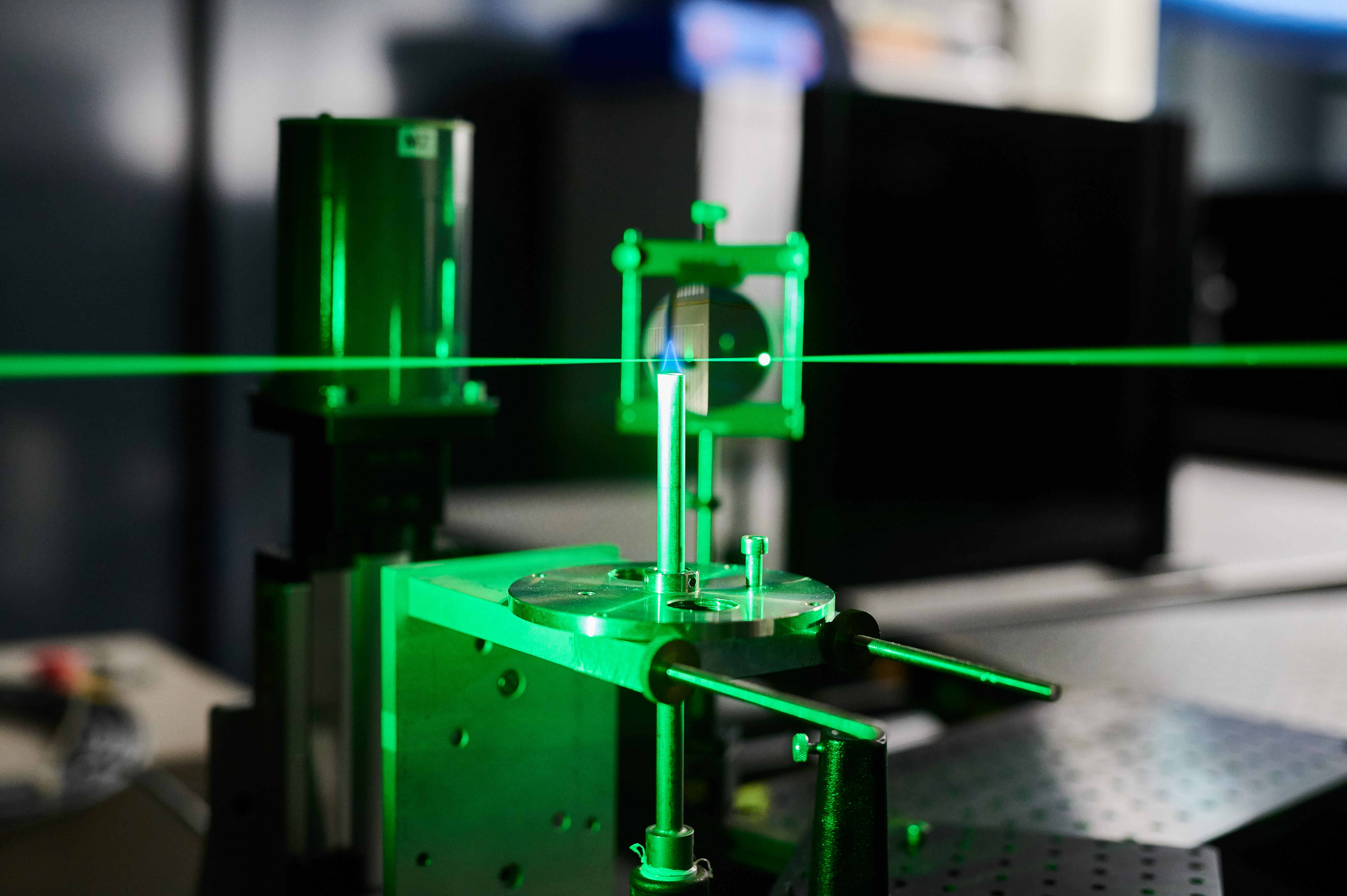
We consider that it is essential to understand the microscopic details of fuel conversion processes for modelling combustion systems, fuel cell systems and electrolyser systems . Towards this end, the group studies elementary physical and chemical processes in high temperature multiphase environments such as flames and plasma’s employing state-of-the-art, laser-based measurement techniques. In particular, we use laser-induced fluorescence (LIF), spontaneous Raman scattering, coherent anti-Stokes Raman scattering (CARS), laser-induced incandescence (LII) and various absorption methods such as direct tunable diode laser absorption (TDLAS) and cavity ring-down spectroscopy (CRDS). Studies of combustion processes are performed using laboratory burner setups at atmospheric and reduced pressures, while a rapid compression machine (RCM) is used to study transient phenomena such as spontaneous ignition. Together with the theoretical and numerical analysis of fuel conversion processes, the results are used to develop efficient and clean industrial and domestic appliances. Electrochemical impedance analysis, surface studies, and other electrochemical lab techniques are employed to study fuel oxidation electrochemistry and electrolysis systems. Fuel oxidation studies often lead to fuel processing efforts including fuel gas cleaning.
System thermodynamic studies are carried out in order to come up with efficient system concepts. Exergy analysis is often employed. These studies are at times followed up with techno-economic and environmental impact analysis in order to come up with viable solutions for energy related technical challenges. Integrated system development, testing and system control efforts are also regularly taken up.
Combining all these, broadly speaking, we develop the concepts for future energy conversion systems. We attempt to contribute significantly to the development of ultra-high efficiency power plants, gasifier- fuel cell systems, reversible power plants, hydrogen fuelled energy systems, future fuels and fuel systems, carbon neutral and negative emission futures.
| Last modified: | 01 December 2022 4.55 p.m. |