Life-emulating molecules show basic metabolism
(There is also a Dutch version of this article)
In a system with self-replicating molecules – previously shown to have the capability to grow, divide and evolve – chemists from the University of Groningen have now discovered catalytic capabilities that result in a basic metabolism. Furthermore, they linked a light-sensitive dye to the molecules, which enabled them to use light energy to power growth. These findings, which bring artificial life one step closer, were published simultaneously in the journals Nature Chemistry and Nature Catalysis on 26 June.
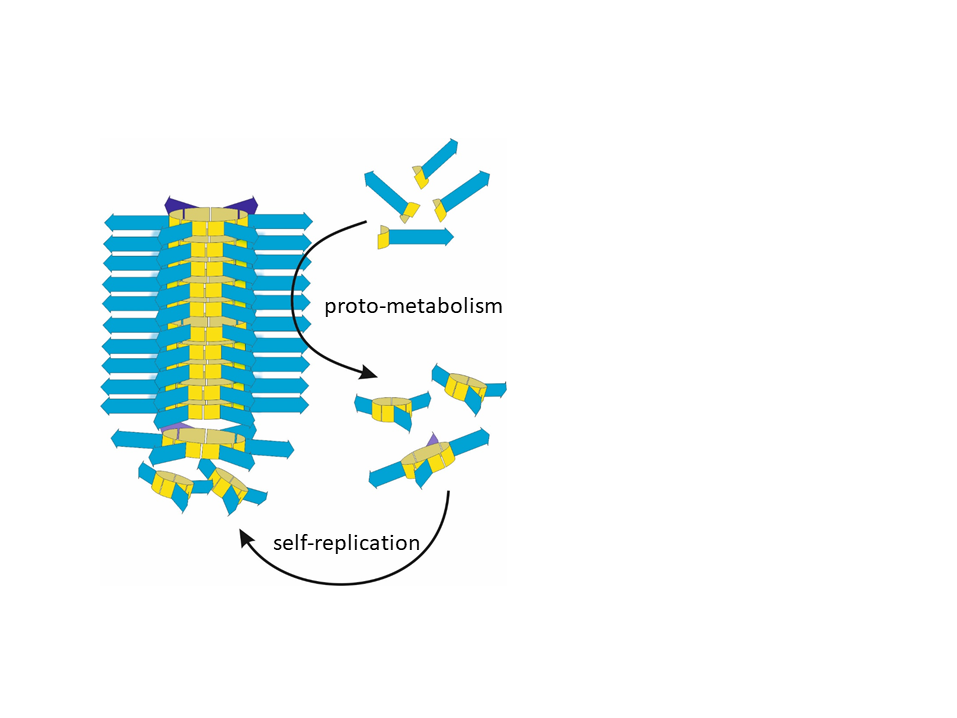
Ten years ago, Sijbren Otto, Professor of Systems Chemistry at the University of Groningen’s Stratingh Institute for Chemistry, discovered a new mechanism for self-replication: small peptide-containing molecules in solution form rings that subsequently form growing stacks. When a stack breaks, both halves start to grow again. Furthermore, the growth of stacks depletes the number of rings in solution and this in turn stimulates the formation of new rings from the building blocks. The system could also ‘mutate’ when different building blocks were added.

Stunning discovery
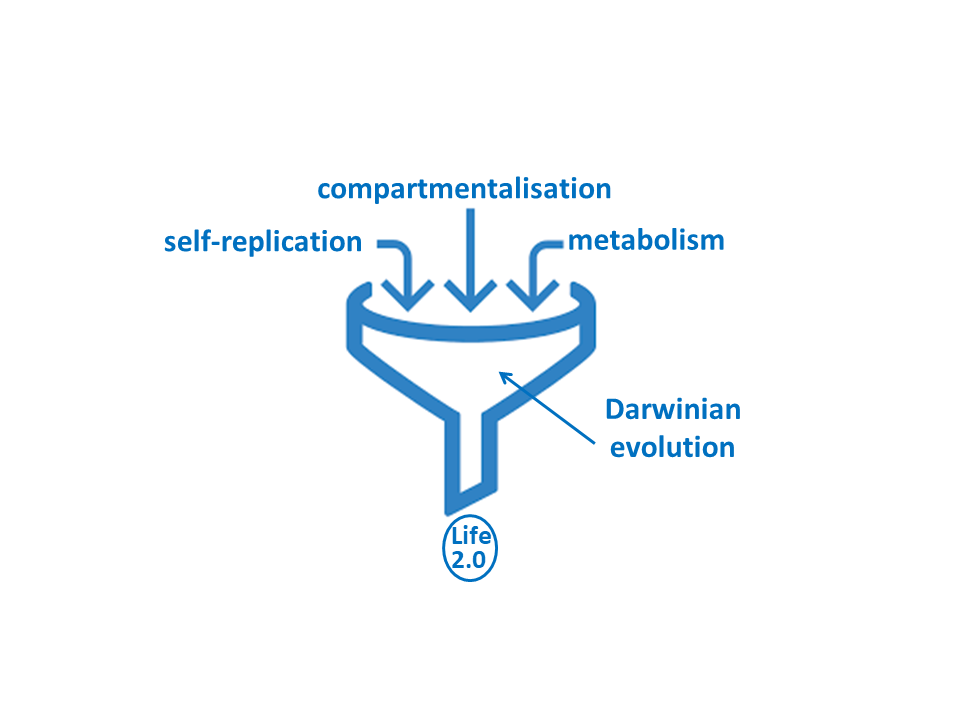
This system, which arose spontaneously, is a form of artificial proto-life. ‘The definition of life is complex but in general life should have three basic properties,’ explains Otto. ‘The first is replication, and this happens in our system. The second is metabolism, which should create building blocks from materials in the environment. And the third is compartmentalization, which separates the living organism from its surroundings.’ Finally, such organisms should develop a fourth, more advanced property, which is the ability to evolve and invent.
Otto and his team set out to make changes to their molecules in order to add catalytic capabilities. ‘However, when we started the project, we made a stunning discovery. Without requiring any changes, the system already showed catalysis; we just hadn’t noticed this before.’ The stacks grow from rings made up of six building blocks. These rings are formed by combining the building blocks of smaller rings that are made up of three or four building blocks.
Evolution
‘It turned out that the stacks of rings catalyse the formation of the smaller rings,’ says Otto. Further analysis showed that catalysis of this reaction requires the presence of two specific amino acid residues (two lysine residues). ‘Neither the building blocks, nor the separate rings have catalytic abilities but the stacks do. So, we assume that in these stacks, a 3D configuration of these lysine residues arises that acts as the catalytic centre, just like proteins shape active sites by placing amino acid residues in highly specific arrangements,’ explains Otto. Thus, in the structures that emerge as a result of their ability to self-replicate, amino acids become organized in such a way that they can act as catalysts.
The stacks are also capable of retro-aldol catalysis, a well-known reaction that is often used to benchmark catalyst design efforts. ‘Interestingly, our stacks, which were not designed to have catalytic capabilities, were as efficient as the best designed catalysts we know.’ Finding out that the same stacks can catalyse two very different reactions is interesting. Many enzymes have this ability, which gives evolution a chance to develop something new.
Metabolism
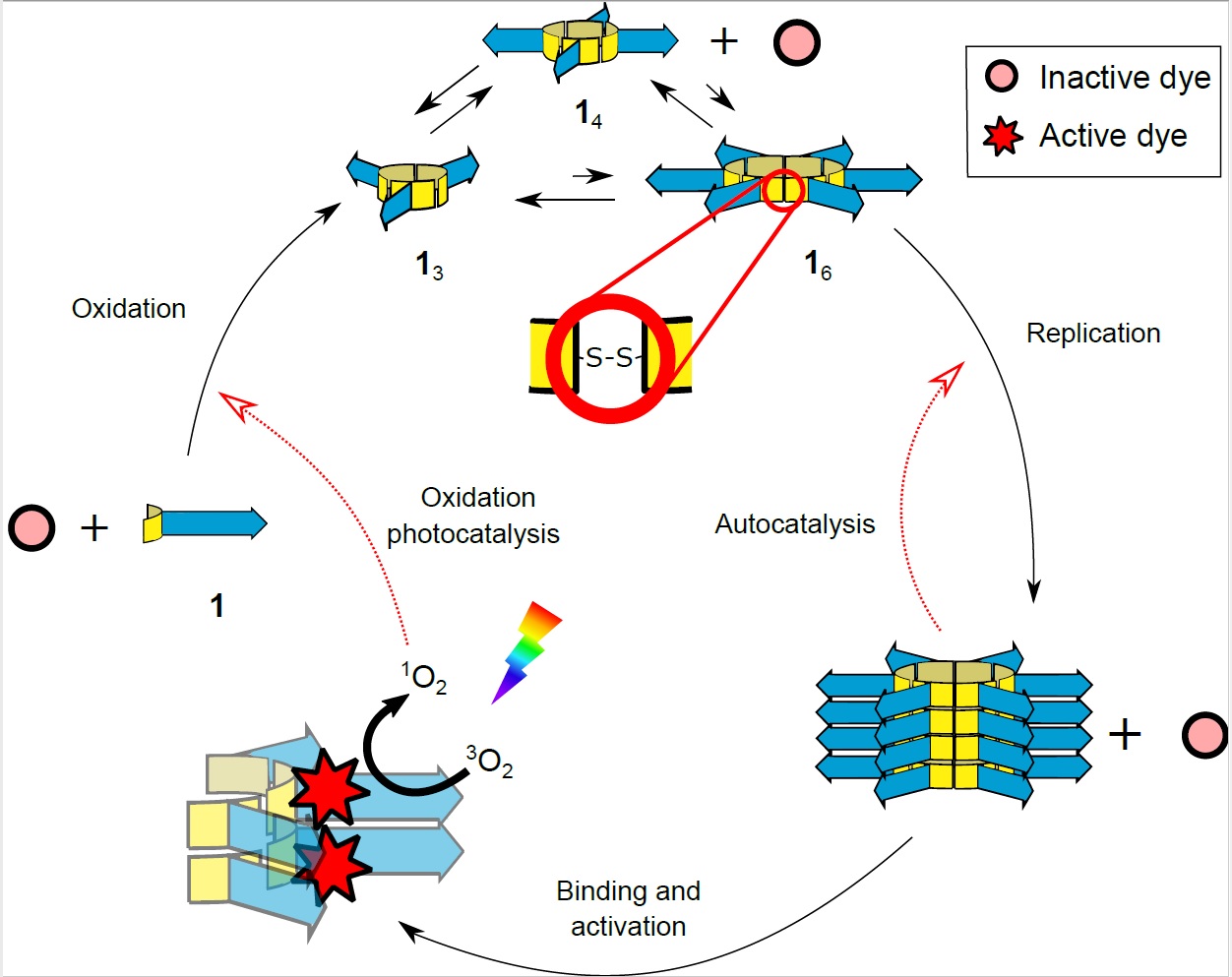
In a second study, a photosensitive dye was added. ‘Guille Monreal, one of my PhD students, read that such a dye could stimulate the formation of reactive singlet oxygen in amyloid peptides. As reactive oxygen drives important steps in ring formation, he wanted to see if this would speed up the formation of rings.’ Two different dyes were found that indeed speed up ring formation when exposed to light, but only when they were bound to the stacks. ‘The dyes appeared to act as cofactors for the stacks, just like modern-day proteins use cofactors for their catalysis,’ says Otto. When bound to the replicating fibres, the dye can use energy from light to create reactive singlet oxygen and thereby increase the formation of new rings.
Both the spontaneous catalysis by the stacks and the catalysis mediated by the cofactor result in a kind of metabolism that is linked to replication. ‘It is not yet the kind of metabolism you see in living organisms,’ explains Otto. ‘In our system, catalysis merely speeds up reactions that would occur slowly without help. In life, metabolism also drives reactions that would otherwise not occur.’
Artificial life
However, Otto’s artificial system shows both replication and a primitive form of metabolism. ‘Furthermore, from this point, compartmentalization is a relatively small step.’ So, is he close to seeing artificial life evolve in his test tubes? ‘Not quite,’ admits Otto. ‘That would require the system to be capable of open-ended evolution, which means it can evolve capabilities that are not present in the system. And we have as yet no clear idea how to accomplish that. But our system appears to be a sound basis from which we may get there.’
A video on this research project can be seen here:
References:
- Jim Ottelé, Andreas S. Hussain, Clemens Mayer, Sijbren Otto: Chance Emergence of Catalytic Activity and Promiscuity in a Self-Replicator. Nature Catalysis 26 June 2020. DOI 10.1038/s41929-020-0463-8
- Guillermo Monreal Santiago, Kai Liu, Wesley R. Browne, Sijbren Otto: Emergence of light-driven protometabolism upon recruitment of a photocatalytic cofactor by a self-replicator. Nature Chemistry 26 June 2020. DOI 10.1038/s41557-020-0494-4
| Last modified: | 29 June 2020 10.10 a.m. |
More news
-
25 April 2025
Leading microbiologist Arnold Driessen honoured
On 25 April 2025, Arnold Driessen (Horst, the Netherlands, 1958) received a Royal Decoration. Driessen is Professor of Molecular Microbiology and chair of the Molecular Microbiology research department of the Faculty of Science and Engineering at the...
-
24 April 2025
Highlighted papers April 2025
The antimalarial drug mefloquine could help treat genetic diseases such as cystic fibrosis, Duchenne muscular dystrophy, as well as some cancers.
-
22 April 2025
Microplastics and their effects on the human body
Professor of Respiratory Immunology Barbro Melgert has discovered how microplastics affect the lungs and can explain how to reduce our exposure.
