A chemically powered unidirectional rotary molecular motor based on a palladium redox cycle

In Nature Chemistry of 8 June 2016 the research group of Ben Feringa presents the design of an organopalladium-based motor and the experimental demonstration of a 360° unidirectional rotary cycle using simple chemical fuels. Exploiting fundamental reactivity principles in organometallic chemistry enables control of directional rotation and offers the potential of harnessing the wealth of opportunities offered by transition-metal-based catalytic conversions to drive motion and dynamic functions.
The conversion of chemical energy to drive directional motion at the molecular level allows biological systems, ranging from subcellular components to whole organisms, to perform a myriad of dynamic functions and respond to changes in the environment. Directional movement has been demonstrated in artificial molecular systems, but the fundamental motif of unidirectional rotary motion along a single-bond rotary axle induced by metal-catalysed transformation of chemical fuels has not been realized, and the challenge is to couple the metal-centred redox processes to stepwise changes in conformation to arrive at a full unidirectional rotary cycle.
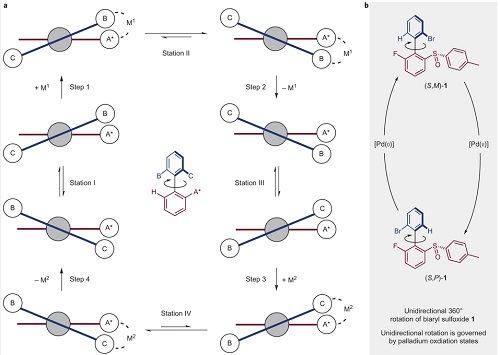
a, Blueprint for a 360° unidirectional rotary molecular motor. The upper aryl ring (the ‘rotor’, blue) rotates through 360° in a clockwise sense with respect to the lower aryl ring (the ‘stator’, red). Unidirectional rotation is governed by selective binding of a metal centre and the formation of diastereomeric metal complexes. b, Proposed realization of this system through the combination of biaryl sulfoxide 1 featuring axial and central chirality and a simple palladium salt allowing shuttling between Pd(0) and Pd(II) redox states.
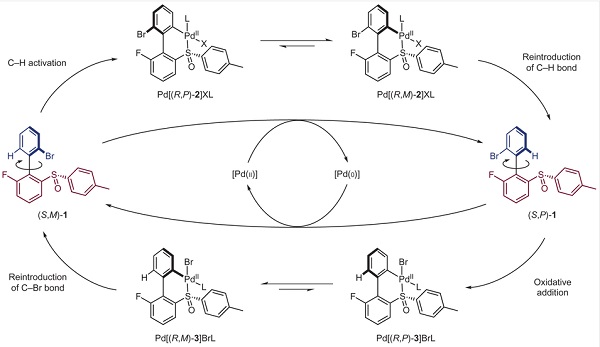
The upper aryl ring (the ‘rotor’, blue) rotates through 360° in a clockwise sense with respect to the lower aryl ring (the ‘stator’, red). Four key steps mediate the rotation: (i) C–H activation; (ii) reintroduction of the C–H bond; (iii) oxidative addition; and (iv) reintroduction of the C–Br bond.
Reference: Beatrice S. L. Collins, Jos C. M. Kistemaker, Edwin Otten & Ben L. Feringa, A chemically powered unidirectional rotary molecular motor based on a palladium redox cycle. Nature Chemistry, 6 June 2016, DOI 10.1038/nchem.2543
Text: Nature.com
| Last modified: | 03 March 2025 09.08 a.m. |
More news
-
01 April 2025
NSC’s electoral reform plan may have unwanted consequences
The new voting system, proposed by minister Uitermark, could jeopardize the fundamental principle of proportional representation, says Davide Grossi, Professor of Collective Decision Making and Computation at the University of Groningen
-
01 April 2025
‘AiNed’ National Growth Fund grant for speeding adoption of AI at SMEs
Professor Ming Cao receives an ‘AiNed’ Growth Fund grant of EUR 2.4 million for research that will contribute to faster adoption of AI at SMEs in the technical industry in the Netherlands.
-
01 April 2025
'Diversity leads to better science'
In addition to her biological research on ageing, Hannah Dugdale also studies disparities relating to diversity in science. Thanks to the latter, she is one of the two 2024 laureates of the Athena Award, an NWO prize for successful and inspiring...
