First look at protein complex regulating cell division
Cell biologists knew it had to be there, but weren’t able to prove that i t was: an elusive protein complex responsible for cell division in bacteria, the divisome. Now two biochemists from the University of Groningen offer a first direct look at the divisome in a paper published on 8 December in Scientific Reports.

Some ten different proteins are involved in the divisome, and PhD student Erik Trip and his supervisor, Professor Dirk-Jan Scheffers, have now found a protein complex containing at least seven of them. ‘So far, scientists have only been able to confirm interactions between two or three proteins from the divisome’, says Scheffers.
‘We first tried to isolate it by using an enrichment step, turning bacterial cells into vesicles.’ They thought this would increase the number of divisome complexes that are associated with the cell membrane. But it didn’t work. The reason for the lack of success so far is simple: the divisome is very fragile.
Scheffers: ‘Once we realized this, we adopted an approach that would minimize damage to the complex.’ Trip grew Escherichia coli cells, and gave them a very mild treatment with enzymes and detergent to release the proteins. The samples were then submitted to a standard technique which separates proteins by size, gel electrophoresis. ‘With this very mild protocol, we found a large protein complex with a weight of one million Dalton.’ The Dalton is a standard measure for protein size.
To confirm that this was indeed the divisome, they used a set of antibodies that bind specifically to the different proteins involved in the complex. These showed that at least seven of the ten were present. Enough to convince the reviewers that they had finally found direct proof for the existence of the divisome.
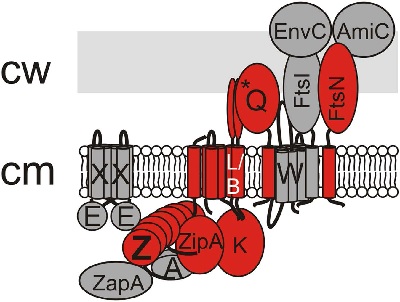
‘Other scientists had inferred that it was there, from genetics studies and interactions between the proposed components, but we are the first to show that it really exists.’ In hindsight, it is not surprising that the divisome was so very fragile. ‘It promotes cell division, so it should disappear rather quickly. Otherwise cells will keep splitting off bits of the cell membrane.’
The divisome only appears when conditions are perfect. This has implications for Scheffers’ colleagues who are trying to build an artificial cell. ‘Our paper shows that it will be very difficult to create a stable divisome. It breaks down very quickly.’
On a positive note, the divisome may be an interesting target for antibiotics. ‘All bacteria have a very similar divisome, which is different from the complex used by plant and animal cells. So if we could disrupt the divisome, that would selectively stop the growth of bacteria.’ He is using this approach in a project aimed at fighting the bacterium which causes citrus canker, a disease which is killing off citrus trees in different parts of the world.
Reference: A 1 MDa protein complex containing critical components of the Escherichia coli divisome. Erik N. Trip & Dirk-Jan Scheffers, Scientific Reports, DOI 10.1038/srep18190
| Last modified: | 13 March 2020 02.10 a.m. |
More news
-
30 January 2025
Highlighted papers December 2024 - January 2025
Read our highlighted papers from December/January: new insights into electronics from 2D materials, and into the protein clumps that cause Huntington's disease.
-
28 January 2025
Studying the universe to understand the world
By understanding the cosmos, we can better fathom the fundamentals of our world. That is the idea behind the research theme Fundamentals of the Universe, in which three institutes of the University of Groningen form a unique collaboration.
-
27 January 2025
Working on better AI (with a smaller budget than the US)
The US is going to invest a staggering amount of $500 billion in AI. At the University of Groningen, researchers are working on future-proof computing: more energy-efficient hardware and responsible AI that can collaborate with humans.
