Hydrogen as an indirect greenhouse gas

Hydrogen is an indirect greenhouse gas: by reacting with other compounds in the atmosphere, it may contribute to global warming in several ways. That is why researchers of the research group led by Professor of Isotope Physics Harro Meijer of the University of Groningen measure hydrogen leaks at Chemical Park Delfzijl.
FSE Science Newsroom | Charlotte Vlek
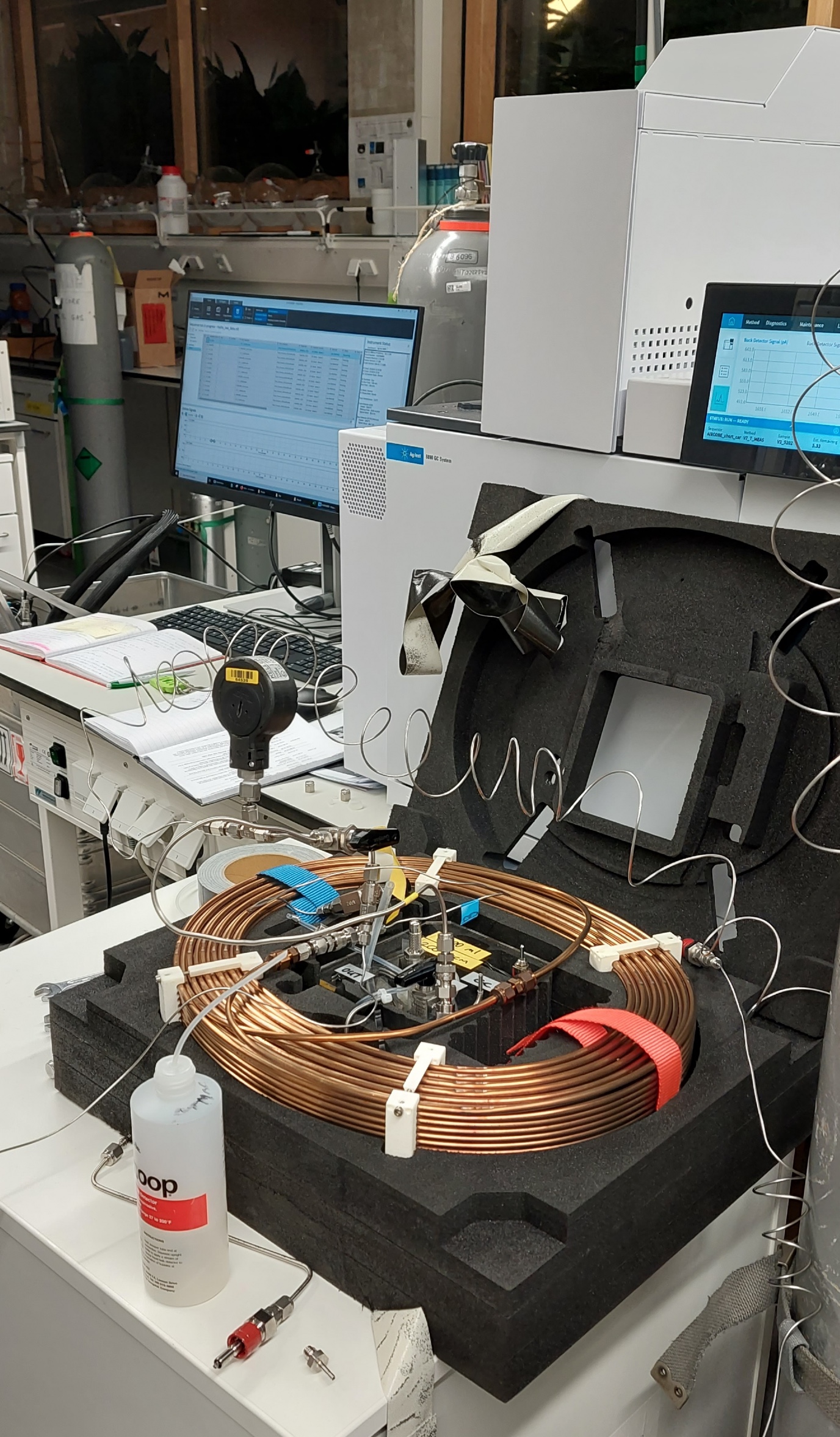
With something that looks like a coiled garden hose in a box in the back seat, PhD student Iris Westra and researcher and lab coordinator Bert Scheeren drive to Chemical Park Delfzijl to map out the concentration of hydrogen. The chemical park is home to several companies that work with hydrogen, including a chlorine plant and a hydrogen filling station.

The box on the back seat, the active AirCore, takes small samples of air during the ride and stores them in chronological order in the coiled tube, which is about three hundred metres long. The location data are also stored, so that when Westra and Scheeren return to the lab, they can examine the samples very precisely per location with a gas chromatograph, a device that identifies which substances are present in the air sample.
The gas chromatograph itself is far too big to be carried around, and it also takes too long to perform the analysis: about seven minutes per location. ‘But, thanks to our active AirCore, we can still take mobile measurements, albeit indirectly,’ says Meijer. ‘We even hook the thing up to a drone, which is why there is also a certified drone pilot present here.’


From passive to active AirCore
The active AirCore was developed at the University of Groningen, based on the passive AirCore that was designed to sample only a vertical cylinder of air. ‘That was basically a long, coiled open tube that was released into the air, tied to a balloon,’ says Scheeren. ‘When the balloon bursts at a high altitude, the tube falls down, collecting a cross-section of the air at a particular location.’
In Groningen, the group of Professor Huilin Chen—now affiliated with Nanjing University, China—expanded the system with active air intake technology: by being able to actively suck up air, many more opportunities opened up. The active AirCore is used for various projects: in addition to hydrogen concentrations, it also measures various greenhouse gases at various locations in the Netherlands, for instance.
The measurements
Based on the measurements, Westra and Scheeren create models of hydrogen leaks and of how the hydrogen spreads through the atmosphere. ‘In that respect, Chemical Park Delfzijl is the perfect place,’ the researchers say. ‘It can be considered a kind of island in an environment with no other hydrogen emissions.’ That is why the measured leaks are fairly easy to trace back to their source: the chemical park.
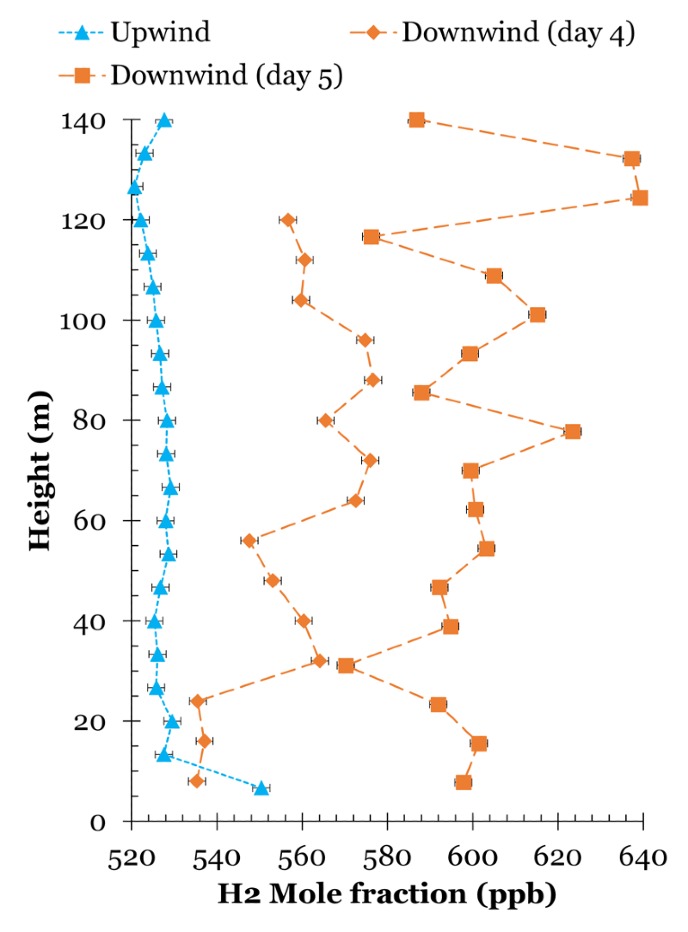
Incidentally, the researchers are not concerned with the chemical park itself, but rather with proving that their measurement method works. And it does that well, Westra says: ‘We have demonstrated that this is a good method to map out hydrogen concentrations. By using precise data on the industry’s daily production, we can then calculate what percentage is leaking. It is just that we do not have the complete data.’
Something is leaking
What we definitely do know is that around the factories and the hydrogen filling station, a lot of hydrogen is leaking into the atmosphere. For example, Westra and Scheeren see a clear difference between the upwind and downwind areas of the chemical park. They also measure an increased concentration of hydrogen at ground level. ‘People always say that hydrogen is such a light substance that it will immediately evaporate,’ Westra says. ‘But that is clearly not the case.’ However, it does not involve such large quantities that it would lead to an explosion hazard, for instance, because that is something the industry itself closely monitors.
Hydrogen: an indirect greenhouse gas
If we start using a lot more hydrogen in industry, this will pose a problem for the climate.
Pure hydrogen in the atmosphere is bad news, the researchers explain. In itself, hydrogen is a clean fuel when it reacts with oxygen in a controlled application, with water vapour as the end product. It becomes problematic when pure hydrogen is leaking and starts reacting in uncontrolled conditions, with other compounds that are present in the atmosphere.
The reaction with the hydroxyl radical (OH) is particularly problematic: this radical normally reacts with the greenhouse gas methane and renders it harmless. If the radical reacts with hydrogen, this no longer happens, leaving more greenhouse gas in the atmosphere. The reaction between hydrogen and hydroxyl radical also leads to more ozone in the atmosphere and more water vapour high in the atmosphere. All three of these lead to global warming, so hydrogen leaking into the atmosphere is not a good thing.
‘For factories, a small loss of hydrogen into the atmosphere is not a problem,’ Scheeren explains. ‘It’s not unsafe; only a concentration of about ten per cent or more presents an explosion hazard. The leakage does not represent a financial burden for the industry either. But if we start using a lot more hydrogen in industry, this kind of leakage will pose a problem for the climate.’
The Hydrogen Valley Campus Europe is located in the Northern Netherlands. It is the place where green energy comes ashore (from wind turbines on the North Sea), where years of working with natural gas has led to a wealth of experience, and where universities and vocational institutions will work on new research and the education of the next generation of engineers for the hydrogen economy of the future. This is the last episode in a series on hydrogen research at the Faculty of Science and Engineering at the University of Groningen.
Read more:
| Published on: | 11 June 2024 |
Because hydrogen is a much smaller molecule than natural gas, it can easily leak. Even worse, despite its small size, hydrogen can affect larger materials and make them as brittle as glass.
| Published on: | 04 June 2024 |
Professor of Energy Conversion Aravind Purushothaman Vellayani is working on systems that use hydrogen to produce electricity – for large factories, for instance. But even your car or your toilet could be capable of producing electricity from hydrogen.
| Published on: | 30 May 2024 |
A sustainable way to produce hydrogen has been around for a century, but it is still much cheaper to make hydrogen from natural gas. Researchers from the UG are working on more efficient, affordable, and scalable production of green hydrogen.
| Published on: | 21 May 2024 |
Green hydrogen holds many promises. But grey hydrogen from natural gas is still much cheaper, storage of hydrogen is not trivial and as an indirect greenhouse gas is not as clean as it might look.
| Last modified: | 13 February 2025 2.15 p.m. |
More news
-
03 April 2025
IMChip and MimeCure in top 10 of the national Academic Startup Competition
Prof. Tamalika Banerjee’s startup IMChip and Prof. Erik Frijlink and Dr. Luke van der Koog’s startup MimeCure have made it into the top 10 of the national Academic Startup Competition.
-
01 April 2025
NSC’s electoral reform plan may have unwanted consequences
The new voting system, proposed by minister Uitermark, could jeopardize the fundamental principle of proportional representation, says Davide Grossi, Professor of Collective Decision Making and Computation at the University of Groningen
-
01 April 2025
'Diversity leads to better science'
In addition to her biological research on ageing, Hannah Dugdale also studies disparities relating to diversity in science. Thanks to the latter, she is one of the two 2024 laureates of the Athena Award, an NWO prize for successful and inspiring...
