University of Groningen chemists produce new-to-nature enzyme containing boron
Boronic acid has been used in organic chemistry for decades, even though it is not present in any organism. ‘It gives rise to different chemical reactions than those we find in nature,’ explains Gerard Roelfes, Professor of Biomolecular Chemistry & Catalysis at the University of Groningen. His group created an enzyme with boronic acid at its reactive centre and then used directed evolution to make it more selective and to improve its catalytic power. Furthermore, enzymatic reactions are more sustainable than classical chemical reactions, as they take place at low temperatures and without toxic solvents. The study was presented online in the journal Nature on 8 May.
FSE Science Newsroom | René Fransen
The application of boron in organic chemistry dates back some seventy years and was awarded a Nobel Prize for Chemistry in 1979. In recent years, the interest in boron as a catalyst has grown, but as yet, its use in the chemical industry is limited. Roelfes: ‘So far, boron catalysis is too slow and it is not very suitable for enantioselective reactions.’ These types of reactions are used to create chiral molecules, which can exist in two versions that are mirror images of each other, like a left and a right hand. In many drugs, both ‘hands’ can have a different effect. It is, therefore, important to selectively produce the proper ‘hand’, especially for the pharmaceutical industry.
(Article continues below the illustration)
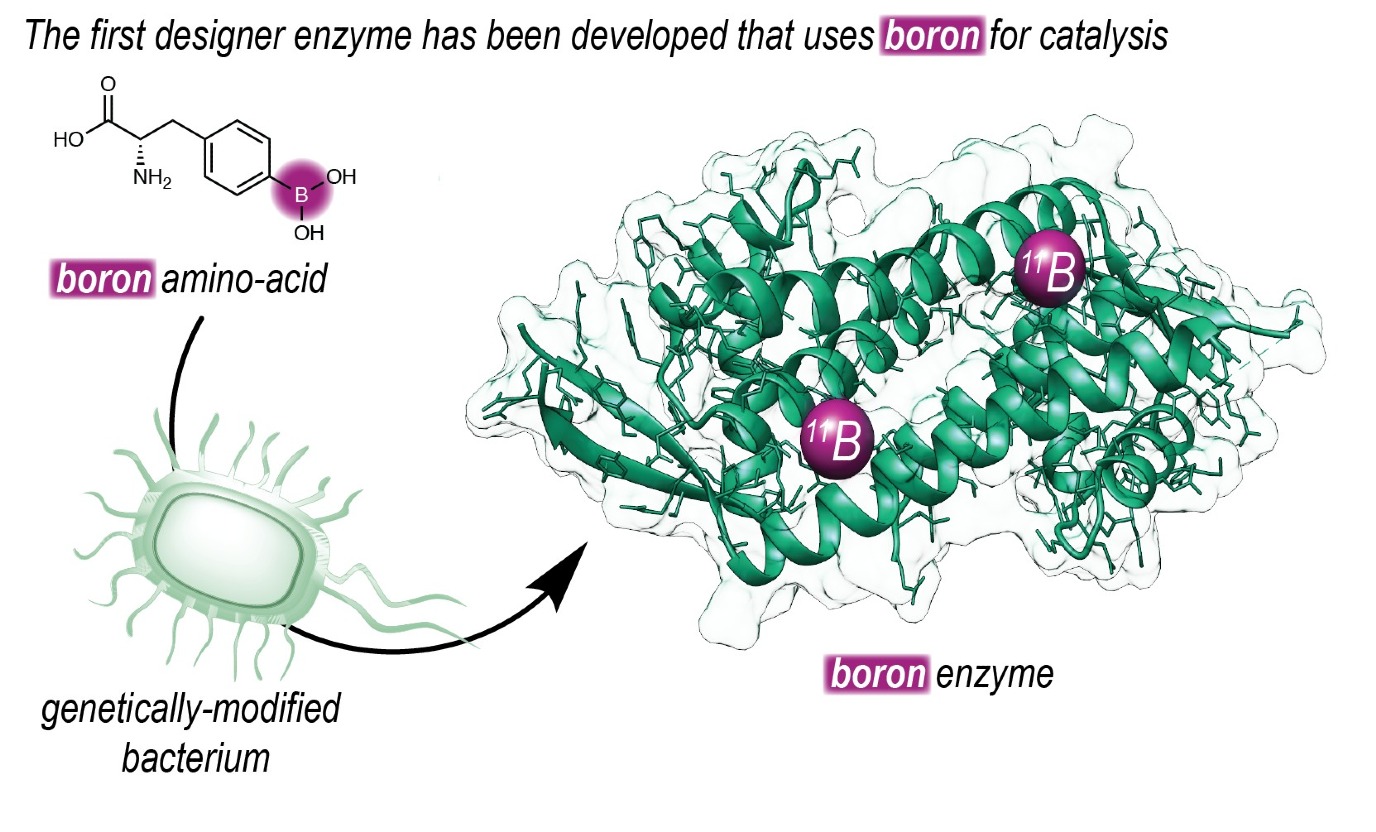
Expanded genetic code
‘To make this possible, we set out to introduce boron into an enzyme. Our group has a long history of designing enzymes that don’t exist in nature.’ The Roelfes group used an expanded genetic code to introduce a non-natural amino acid that contains a reactive boronic acid group into an enzyme. ‘Using this technique, we can determine at the DNA level where we place the amino acid in a protein.’
Once they made an enzyme with boronic acid at its reactive centre, they could use directed evolution to increase its efficiency, resulting in faster catalysis. ‘Furthermore, by placing the boronic acid in the chiral context of an enzyme, we were able to achieve highly enantioselective catalysis.’ The reaction that is described in the journal Nature is a ‘proof of principle’ and shows the way to harnessing the catalytic power of boron in enzymes.

Biocatalysis
Using enzymes to create organic compounds is important for the pharmaceutical industry. ‘In their push towards greener and more sustainable ways of producing drugs, they are looking at biocatalysis to replace conventional chemical reactions.’ At the University of Groningen, concerted efforts are being made towards this goal. ‘We have a number of research groups at the Faculty of Science and Engineering engaged in this kind of work, using different approaches to create biocatalytic solutions for the chemical industry.’ In this context, Roelfes and his team will continue to develop their boronic acid enzymes and create other such new-to-nature enzymes.
Reference: Lars Longwitz, Reuben B. Leveson-Gower, Henriëtte J. Rozeboom, Andy-Mark W. H. Thunnissen & Gerard Roelfes: Boron catalysis in a designer enzyme. Nature, online 8 May 2024.
| Last modified: | 03 October 2024 10.23 a.m. |
More news
-
03 April 2025
IMChip and MimeCure in top 10 of the national Academic Startup Competition
Prof. Tamalika Banerjee’s startup IMChip and Prof. Erik Frijlink and Dr. Luke van der Koog’s startup MimeCure have made it into the top 10 of the national Academic Startup Competition.
-
01 April 2025
NSC’s electoral reform plan may have unwanted consequences
The new voting system, proposed by minister Uitermark, could jeopardize the fundamental principle of proportional representation, says Davide Grossi, Professor of Collective Decision Making and Computation at the University of Groningen
-
01 April 2025
'Diversity leads to better science'
In addition to her biological research on ageing, Hannah Dugdale also studies disparities relating to diversity in science. Thanks to the latter, she is one of the two 2024 laureates of the Athena Award, an NWO prize for successful and inspiring...
